Understanding 21 CFR Part 11 – Electronic Records and Electronic Signatures
By Deb Bartel,
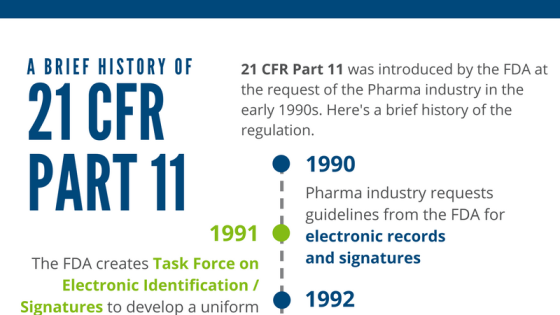
Life Sciences companies that want to sell their products or services in the United States must comply with the 21 CFR Part 11, Electronic Records and Electronic Signatures regulation. This includes systems used in researching, manufacturing, and distributing product such as pharmaceuticals, medical devices, biological products (e.g. vaccines), blood, and tissue (e.g. organ transplants). It is critical that the professionals who … Read more

