Validation Templates and Software Quality SOPs
The Validation Center™ library offers computer system validation SOPs and templates to expedite your implementation of a software validation program that complies with the expectations of the FDA, EMA, and ICH. These SOPs and templates also incorporate industry standards and best practices, such as those found in PIC/S and GAMP.
Showing 1–16 of 43 results
-

Package – Validation Templates and Quality SOPs – Complete Package
Get the complete package of 38 validation templates and quality assurance SOPs for $960. A 60% savings over individual purchases.
$960.00 Add to cart -

Package – Software Quality Assurance SOPs
Get a full suite of 17 software quality assurance SOPs for $690. A 45% savings over individual purchases.
$690.00 Add to cart -

Package – Validation Templates and SOP – Basic Package
Get a basic package of 11 validation templates and a CSV SOP for $595. A 30% savings over individual purchases.
$595.00 Add to cart -
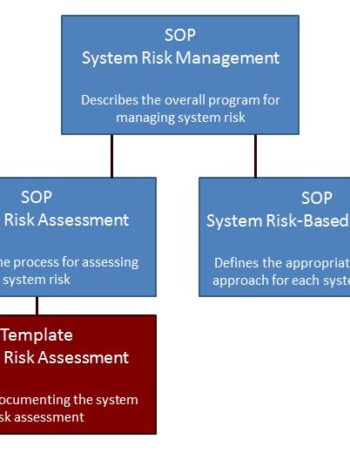
Package – Risk Management
Get 3 risk management SOPs and a risk assessment template for $240. A 20% savings over individual purchases.
$240.00 Add to cart -

Package – Software Vendor Assessment
Get the Software Vendor Assessment SOP and template for $120. A 20% savings over individual purchases.
$120.00 Add to cart -

CSV Template – 21 CFR Part 11 Assessment
This template documents the assessment of a system for compliance to 21 CFR Part 11. Template includes 37 questions with cross-references to 21 CFR Part 11.
$75.00 Add to cart -

CSV Template – Computer System Risk Assessment
This form is used to document the system-level and major function-level risk assessment in terms of criticality and complexity.
$25.00 Add to cart -

CSV Template – Functional Requirements Specification
The FRS documents the functionality required for intended use of the system. It contains detailed feature list and will be used in development, configuration, and test creation.
$75.00 Add to cart -

CSV Template – Installation Qualification (IQ)
The IQ documents the installation, configuration and associated verification of a system, system components, or supporting peripherals.
$75.00 Add to cart -
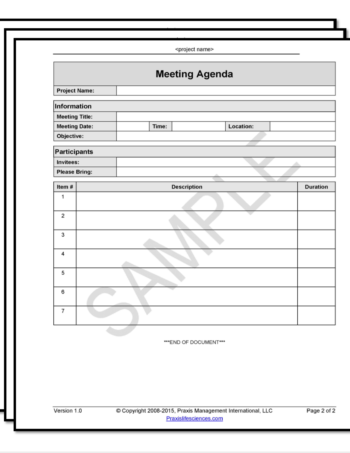
CSV Template – Meeting Agenda
The meeting agenda template provides a quick mechanism to communicate the key elements and logistics of a meeting.
$0.00 Add to cart -

CSV Template – Meeting Minutes
Meeting minutes are a key communication tool for any project. This template provides an easy format for recording attendance, discussion, activities, and action items.
$0.00 Add to cart -

CSV Template – Periodic Validation Review Report
The Periodic Validation Review (PVR) Report template SOP is used to document the assessment of a system’s current validation state and plan for closure of any gaps.
$75.00 Add to cart -

CSV Template – Project Plan
The Project Plan template documents the project’s business case, scope, deliverables, organization, budget, milestones, schedule, work breakdown structure, and controls.
$75.00 Add to cart -

CSV Template – Spreadsheet Validation
Spreadsheet Validation Template for concise and compliant documentation of validation plan, activities, and results. Also suitable for validation of reports, queries and calculations.
$75.00 Add to cart -

CSV Template – System Design Document
The System Design documents how the system will meet the FRS through design of system architecture, data structures, data flows, features, and interfaces.
$75.00 Add to cart -

CSV Template – Testing Plan
The Testing Plan is used to document the validation testing methodology, strategy, tools, environments, and responsibilities. It includes the plan for IQs, OQs, and PQs.
$75.00 Add to cart
