Document Library
Showing 1–16 of 179 results
-

Custom Order – Maria Porter
Hard copy of student materials for CSV Boot Camp, including student binder, Therac case study, and handouts binder. Order includes free ground shipping within the US.
$135.88 Add to cart -

Change Fee
We understand that things happen and you may need to change your class date. Our classes have limited space and require upfront purchases, so we have the following change policies:
$100.00 Add to cartDate Rescheduling Policy Greater than 15 days No penalty Within 15 days $100 change fee (must be paid at that time change is made) After start of class Cannot change session date. No refund. - Sale!

Buy 2 Get 1 FREE
Original price was: $1,599.00.$1,499.00Current price is: $1,499.00. Add to cart -

FDA Guidance: Data Integrity and Compliance with Drug CGMP, 12/18
This FDA Guidance outlines the agency’s expectations for data integrity controls such as, records and metadata retention, audit trail reviews, backups, access, unique logins, and record storage location.
$0.00 Add to cart -

PIC/S PI 041-1 Draft Guidance: Good Practices for Data Management and Integrity, 11/18
November 2018, Draft PIC/S data management and integrity guidelines for manufacturers and distributors of APIs and medicinal products.
$0.00 Add to cart -

FDA Guidance: Use of Electronic Health Record Data in Clinical Investigations, 7/18
July 2018 FDA Guidance on the best practices to follow when using electronic health record (EHR) data in clinical investigations.
$0.00 Add to cart -

MHRA GXP Data Integrity Guidance and Definitions, 3/18
This update to the MHRA Data Integrity Guidelines adds new topics, such as Cloud providers, electronic signatures, and data migrations.
$0.00 Add to cart -

FDA Draft Guidance: Current Good Manufacturing Practice for Medical Gases, 6/17
June 2017 Draft FDA Guidance intended to assist manufacturers of medical gases in complying with the regulations within 21 CFR Parts 210 and 211.
$0.00 Add to cart -

FDA Draft Guidance: Use of Electronic Records and Electronic Signatures in Clinical Investigations under Part 11 – Q&A, 6/17
June 2017 Draft FDA Guidance intended to assist study sponsors, investigators, IRBs, and CROs in complying with 21 CFR Part 11, Electronic Records; Electronic Signatures.
$0.00 Add to cart -

Package – Validation Templates and Quality SOPs – Complete Package
Get the complete package of 38 validation templates and quality assurance SOPs for $960. A 60% savings over individual purchases.
$960.00 Add to cart -

Package – Software Quality Assurance SOPs
Get a full suite of 17 software quality assurance SOPs for $690. A 45% savings over individual purchases.
$690.00 Add to cart -

Package – Validation Templates and SOP – Basic Package
Get a basic package of 11 validation templates and a CSV SOP for $595. A 30% savings over individual purchases.
$595.00 Add to cart -
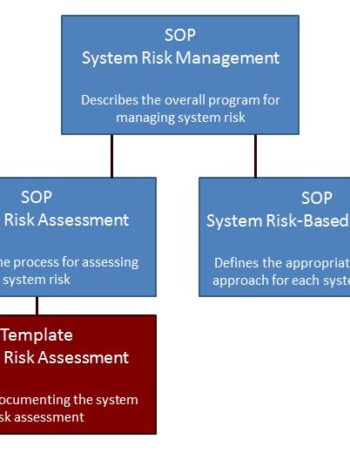
Package – Risk Management
Get 3 risk management SOPs and a risk assessment template for $240. A 20% savings over individual purchases.
$240.00 Add to cart -

Package – Software Vendor Assessment
Get the Software Vendor Assessment SOP and template for $120. A 20% savings over individual purchases.
$120.00 Add to cart -

PIC/S PE 009-13 Guide to Good Manufacturing Practices for Medicinal Products (Introduction), 1/17
Introduction to the January, 2017 version of the PIC/S GMP (good manufacturing practices) Guide. Explains the organization of Parts I and II and the Annexes
$0.00 Add to cart -

PIC/S PE 009-13 Guide to Good Manufacturing Practices for Medicinal Products (Part I), 1/17
In January, 2017 this PIC/S GMP guide went into effect. It includes expectations for documentation and expectations for validation.
$0.00 Add to cart
