Digital Transformation in CSV – How to implement Digital Validation?
By Esra Guven,
The FDA encourages a risk-based approach and critical thinking in Computer Systems Validation and promotes efficiency. Using digital validation tools and adopting paperless validation helps not only meet FDA regulations but also promotes efficiency, consistency, and audit readiness.
“This draft guidance is intended to: Describe ‘computer software assurance‘ as a risk-based approach to establish confidence in the automation used for production and quality systems and identify where additional rigor may be appropriate.” FDA Draft Guidance: Computer Software Assurance for Production and Quality System Software, September 13, 2022, I. Introduction, Page 4
“Advances in digital technology may allow for manufacturers to leverage automated traceability, testing, and the electronic capture of work performed to document the results, reducing the need for manual or paper-based documentation. As a least burdensome method, FDA recommends the use of electronic records, such as system logs, audit trails, and other data generated by the software, as opposed to paper documentation and screenshots, in establishing the record associated with the assurance activities.” FDA Draft Guidance: Computer Software Assurance for Production and Quality System Software, September 13, 2022, D. Establishing the Appropriate Record, Page 19
Let’s look at the benefits of Digital Validation
- Reduces time and effort for all CSV deliverables.
- Ensures consistency and quality during validation.
- Increases accessibility from anywhere at any time.
- Increases collaboration.
- Improves regulatory compliance.
Let’s look at the benefits from ALCOA+ point of view
| Attributable | Enhanced accountability through an audit trail that records who performed each action and when. |
| Legible | Improved clarity of records in human readable form. |
| Contemporaneous | No risk of late recordings. Activities are immediately recorded. |
| Original | Data is properly and directly recorded on the digital platform. |
| Accurate | Enhanced precision with improved format and version control. |
| Complete | All data is present on the digital platform. No risk of selective data or reporting. |
| Consistent | Improved uniformity, with all activities adhering to a standardized approach, minimizing the risk of variation. |
| Enduring | Electronic data is stored on a controlled and backed up storage. |
| Available | Greater accessibility, ensuring data can be reviewed, audited, or inspected throughout the lifetime of the record. |
What are the Core Requirements?
The digital tool for paperless validation must manage both the process and deliverables for CSV. It is the system of record for CSV.
- Must be accessible from anywhere at any time.
- Must allow user to perform risk assessments such as GxP assessment, business risk assessment, system risk assessment.
- Must allow creation of User Requirements and other specification documents.
- Must provide functionality for electronic document creation, review, and approval.
- Must allow user to enter user requirements and map to the test procedures.
- Must provide test development and test execution.
- Must automatically create traceability matrix.
- Must provide functionality for incident management.
- Must allow Administrator to build, modify validation templates
as well as test procedure templates. - Must allow versioning.
- Must have a change management functionality.
- Must provide functionality of electronic signatures.
- Must have Audit trail.
- Must have configurable roles and privileges.
- Must be compliant with regulatory requirements (e.g., 21 CFR Part 11, Annex 11)
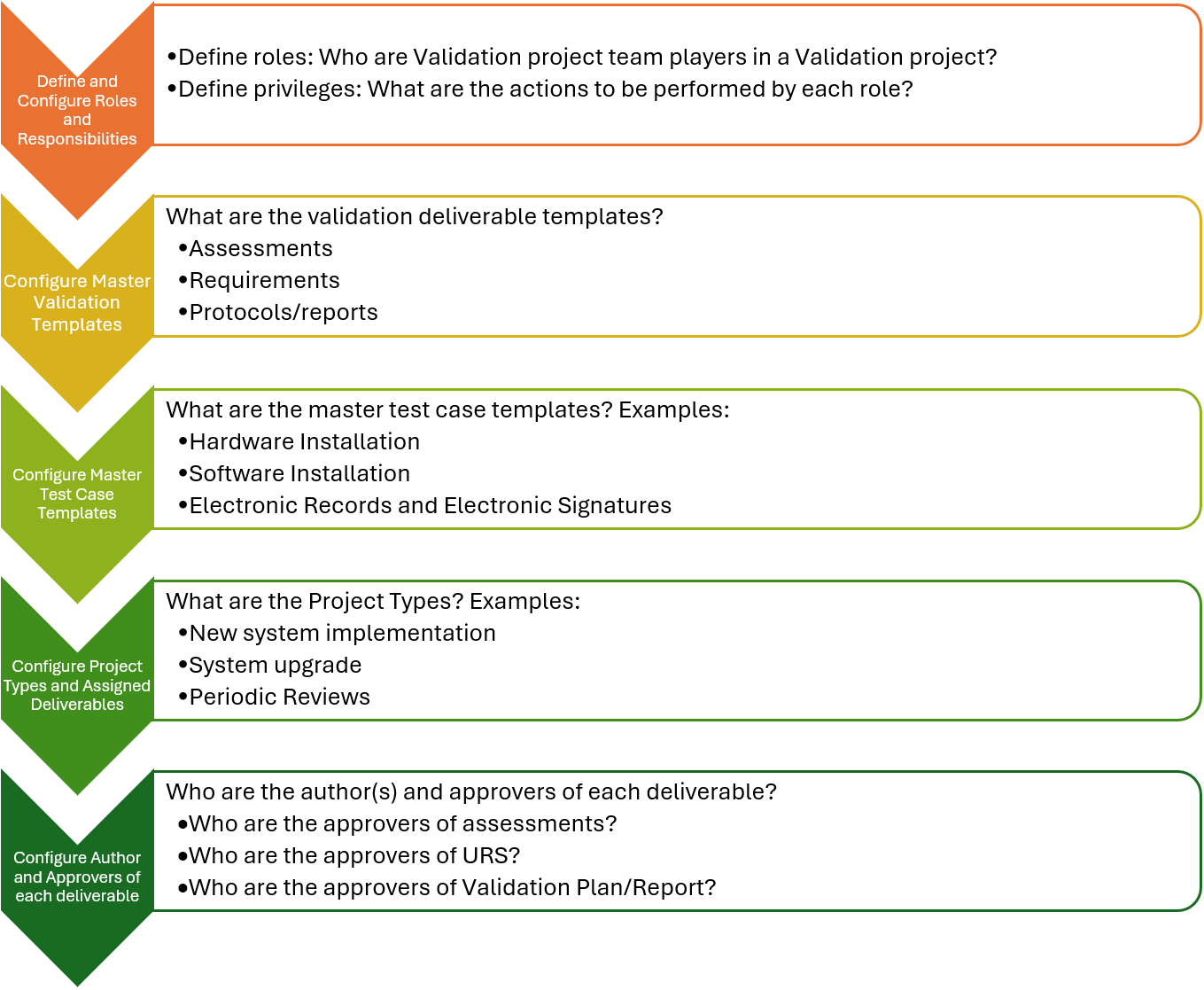
How to Configure a Digital Tool for CSV?
What are the Common Challenges and Pitfalls of a Digital Validation Tool Implementation?
- Overcomplicating the process
- Employee training
- People do not like change
- Poorly defined roles and privileges
- Poor configuration
- Define training requirements for each role defined /configured in the system.
Solutions
WITHOUT COMPROMISING QUALITY AND REGULATORY REQUIREMENTS
- Improve and simplify CSV process during the Digital Platform implementation.
- Define user roles and responsibilities clearly and effectively.
- Reduce approvers. Group the deliverables with the same number of approvers.
- Reduce the number of deliverables covering every aspect of validation.
- Define training requirements for each role configured in the system.
- Create online interactive training and online help in addition to in-classroom training.
- Consider Agile CSA concepts.
SIMPLE AND LEAN
Need more help with Paperless Validation and Implementing Digital tool for CSV activities?
Don’t have the time or expertise on Digital Validation? Let our experienced consultants implement paperless validation.
Copyright Policy
Unless otherwise noted, ProPharma Group Holdings, LLC is the legal copyright holder of all (written, multimedia and graphic) material on this website and it may not be used, reprinted, (partially) modified or published without written consent. A link to Validation Center must appear in all copies of any artwork or content, including articles and press releases.
You are welcome to share this article on social media so long as you link back directly to this post (providing the link). No copy & paste of this content will be allowed under any circumstances.
