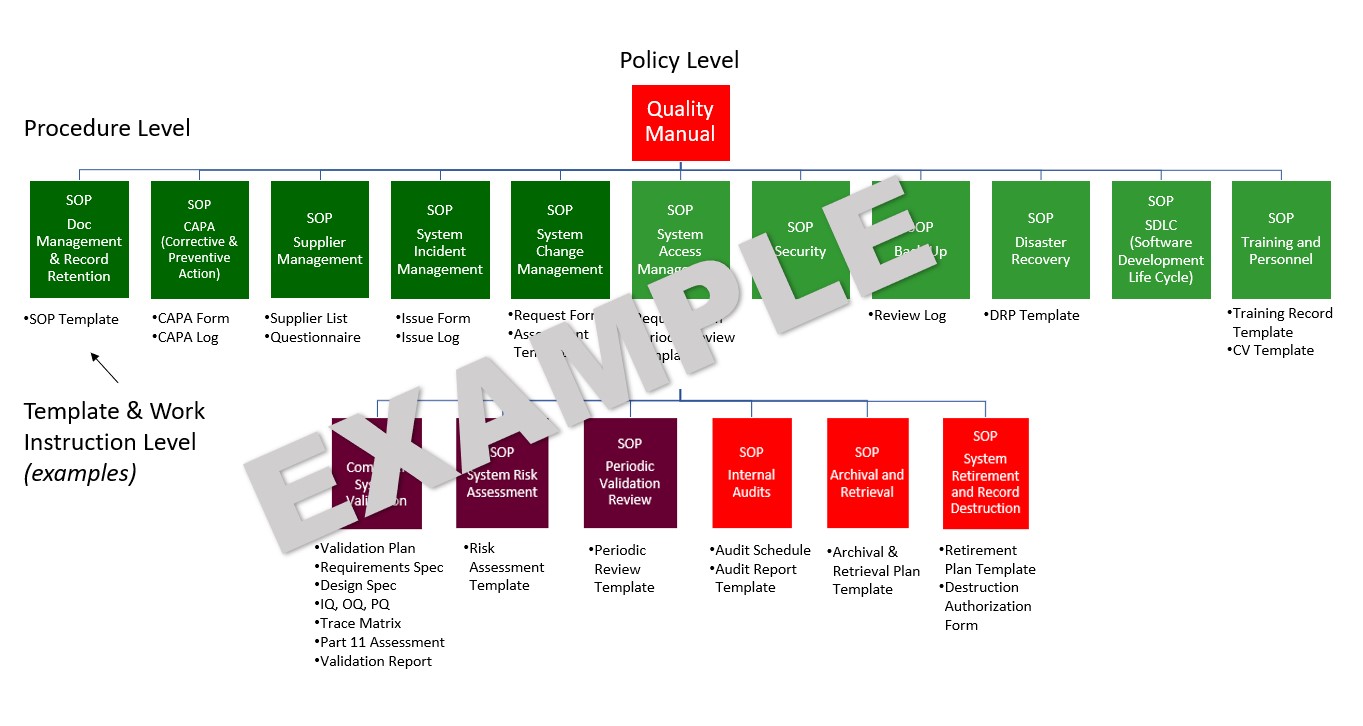
A robust framework of Policies, Procedures, Work Instructions, Templates, and Forms ensures that data integrity is “built-in” from the start.
Our experienced data integrity consultants can have your QMS framework ready in weeks!
Get Help with Policies and Procedures
Policy and Procedure Development
Instead of staring with a blank page, we leverage a pre-built site of SOPs, Templates, and Forms which fully encompass the data integrity requirements documented by the US FDA, EMA, MHRA, WHO, PIC/S, ISPE, and PDA.
Here are the steps we take:
- Meet with you to discuss what makes your organization unique, e.g.,
- Industry-specific regulations, e.g., AOPO, CAP, COLA
- Organization structure, roles, and responsibilities
- Computer systems and tools already in use for records and approvals
- Tailor our standard suite of documents to fit your organization
- Solicit your feedback on the preliminary suite of documents and make adaptations before finalizing the content.
- Develop a Quality Manual to summarize the Policy and Procedure framework and provide an overview for employees, auditors, and inspectors.
Data Integrity Training
Delivering the right level of training at the right time and frequency is critical for ensuring that the principles of data integrity become part of company culture.
We have a catalog of Data Integrity training courses which can be provided on ‘as is’ or tailored to include examples from your daily operations.
- Short training courses on Data Integrity Principles, suitable for Data Integrity program implementation and annual refresher training
- Longer training courses on advanced Data Integrity topics such as:
- ALCOA in Practice
- Data Integrity Roles & Responsibilities
- Data Governance Models
- Digital Signatures
- Computer System Validation